NAMMD – in the process of recovering the delays in the activity of evaluation of the authorization/pharmaceutical inspection files.
NAMMD presents in the table below the situation of the completed authorization dossiers/inspections carried out between February and May 2019, compared to 2018.
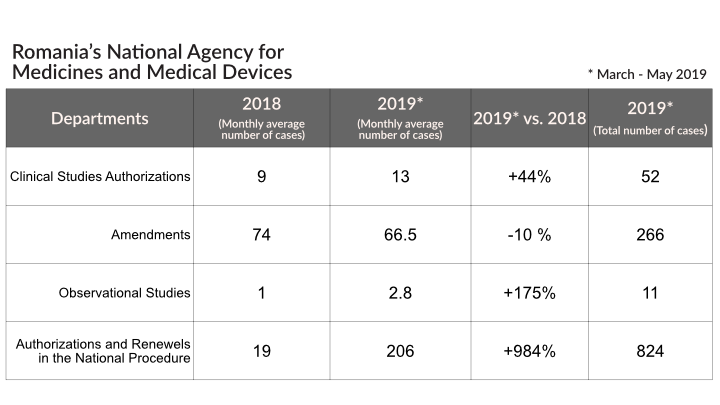
According to their website, the numbers illustrate the intense activity conducted in February – May 2019 vs. 2018, respectively:
- an increase of 44% in the process of authorization of clinical trials;
- an increase of 984% in the national procedure of authorizations and renewals;
- an increase of 175% in the authorization of observational trials.
Taking into account the opening of the NAMMD management to the transparency of the activity, starting from June 2019, the Agency will inform the interested parties every month about the detailed results obtained in the previous month regarding:
- the marketing authorization through the national procedure and the European procedures;
- the authorization for conducting clinical trials;
- evaluation of the files regarding the inclusion in the List of compensated and free medicines, annex to GD 720/2008;
- pharmaceutical inspections to check compliance with Good Distribution Practices and Good Manufacturing Practices.

